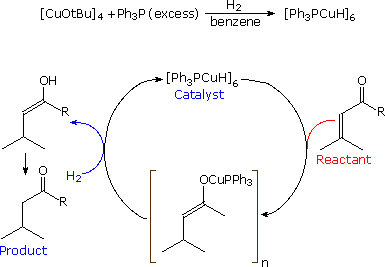
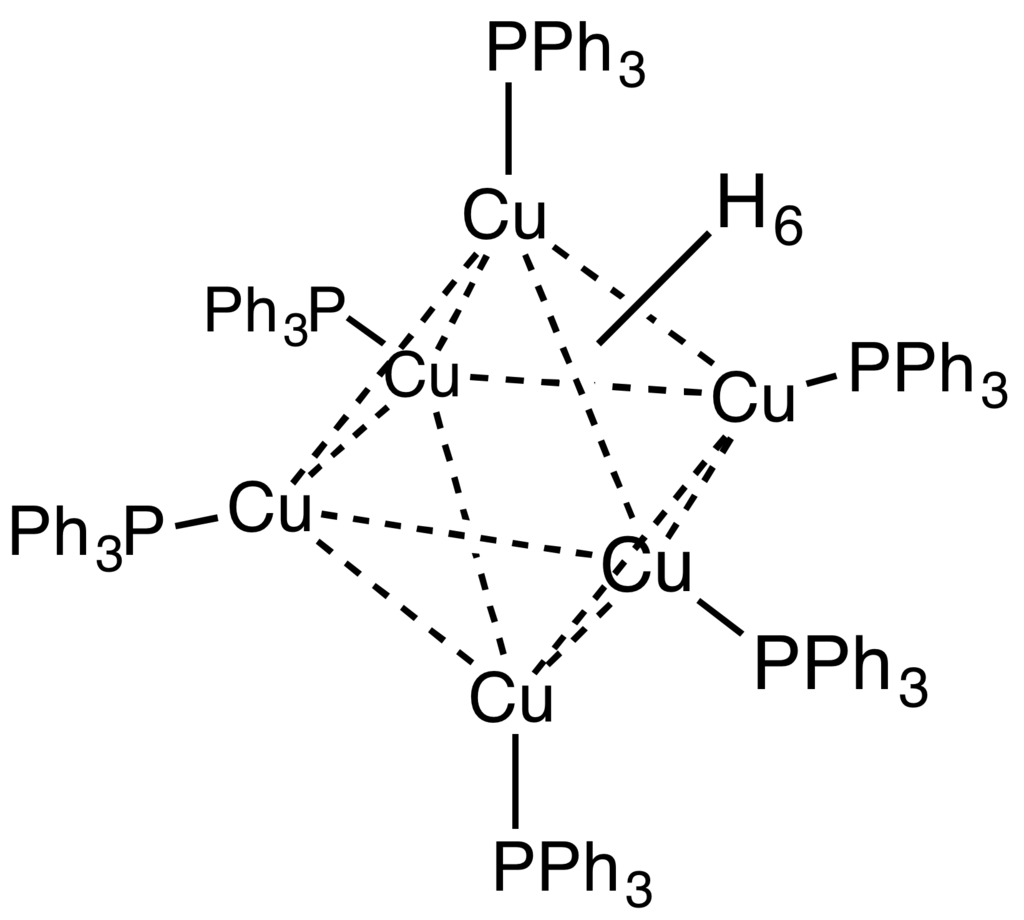
Stryker's reagent ([(PPh3)CuH]6), also known as the Osborn complex, is a hexameric copper hydride ligated with triphenylphosphine. It is a brick red, air-sensitive solid. Stryker's reagent is a mildly hydridic reagent, used in homogeneous catalysis of conjugate reduction reactions of enones, enoates, and related substrates.
The compound can effect regioselective conjugate reductions of various carbonyl derivatives including unsaturated aldehydes, ketones, and esters. This reagent was assigned as the "Reagent of the year" in 1991 for its functional group tolerance, high overall efficiency, and mild reaction conditions in the reduction reactions. Stryker's reagent is used in a catalytic amount where it is regenerated in the reaction in situ using a stoichiometric hydride source, often being molecular hydrogen or silanes. If stored under an inert atmosphere (e.g. argon, nitrogen) it has indefinite shelf life. Brief exposure to the oxygen does not destroy its activity significantly, although solvents used with Stryker's reagent should be rigorously degassed.