Symmetry is beauty. There are countless examples in nature, just think about honeycombs, flowers, starfish or….maleic acid! You probably can guess which of these examples will be the star in this blog post - no, sorry, it is not the starfish (Figure 1).

Figure 1. This blog post is not about starfishes.
Amongst other things, maleic acid is used as an internal calibrant for quantitative NMR (qNMR) analysis. Two of the reasons why maleic acid is such a good internal calibrant are a) the fact that due to its symmetry, maleic acid only shows one singlet in the 1H NMR spectrum (at least when neglecting the 13C satellites and the labile acid proton signal, which does not appear when D2O is added to the sample); and b) that the chemical shift of this singlet is around 6 ppm, a region which otherwise is not too crowded and overlaps with the analyte signals are less likely to occur (Figure 2).
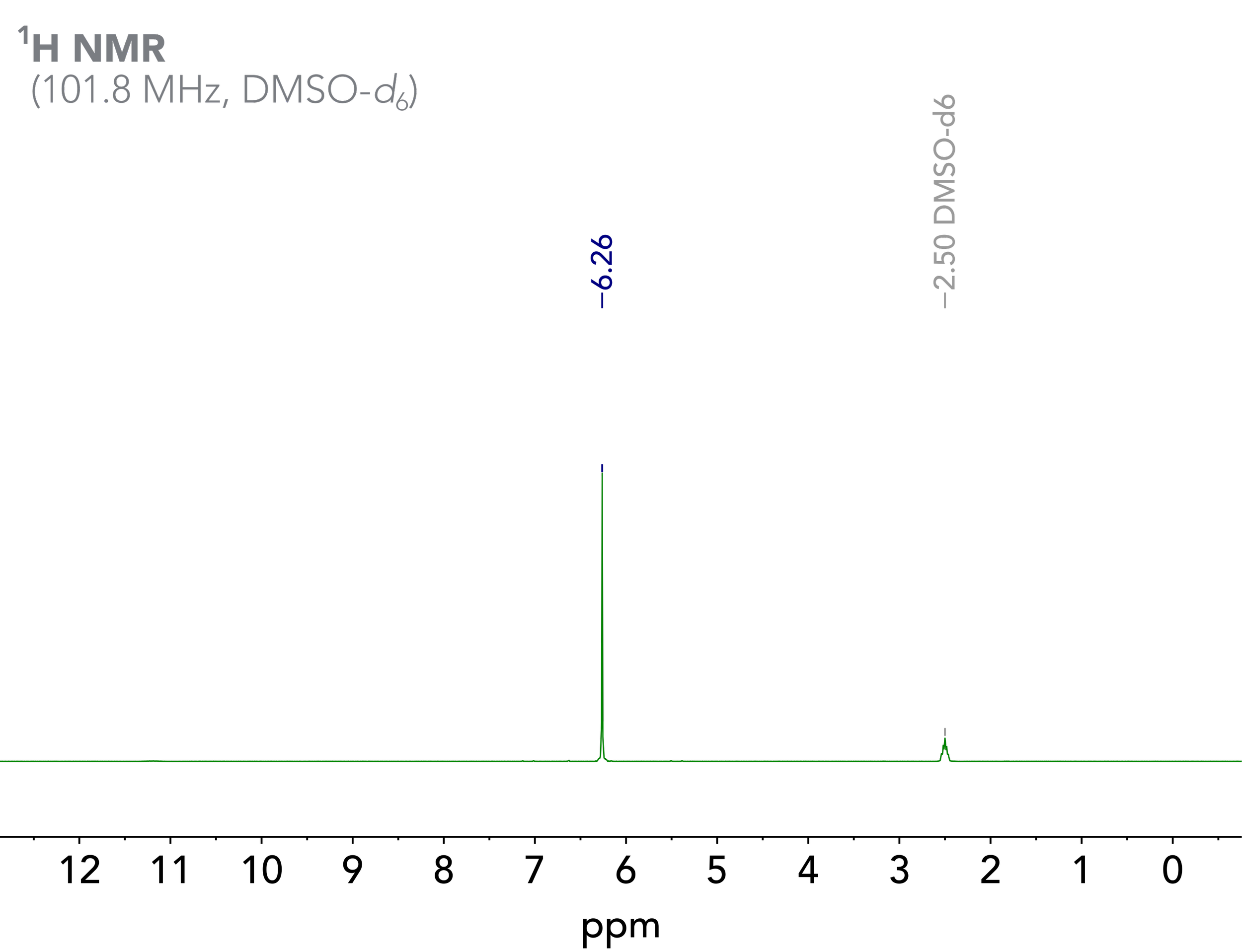
Figure 2. 100 MHz 1H NMR spectrum of maleic acid in DMSO-d6.
We have previously discussed hetero J couplings and unsymmetric carbon satellites in 19F NMR , and I also would like to recommend looking at our infographic on carbon satellites for a good overview on this topic. When looking at the carbon satellites of maleic acid, a lot is going on there (Figure 3). In the zoomed view of the 100 MHz 1H NMR of maleic acid, we observe more peaks than we might have expected. The main species in the spectrum is maleic acid, with all carbon atoms having carbon-12 isotopes (Figure 2, left). This molecule is symmetric, and the two olefinic protons 1 are equivalent, thus we observe a singlet at 6.26 ppm. Remember that 13C is NMR active, and its nuclear spin couples with the one from the directly attached proton.
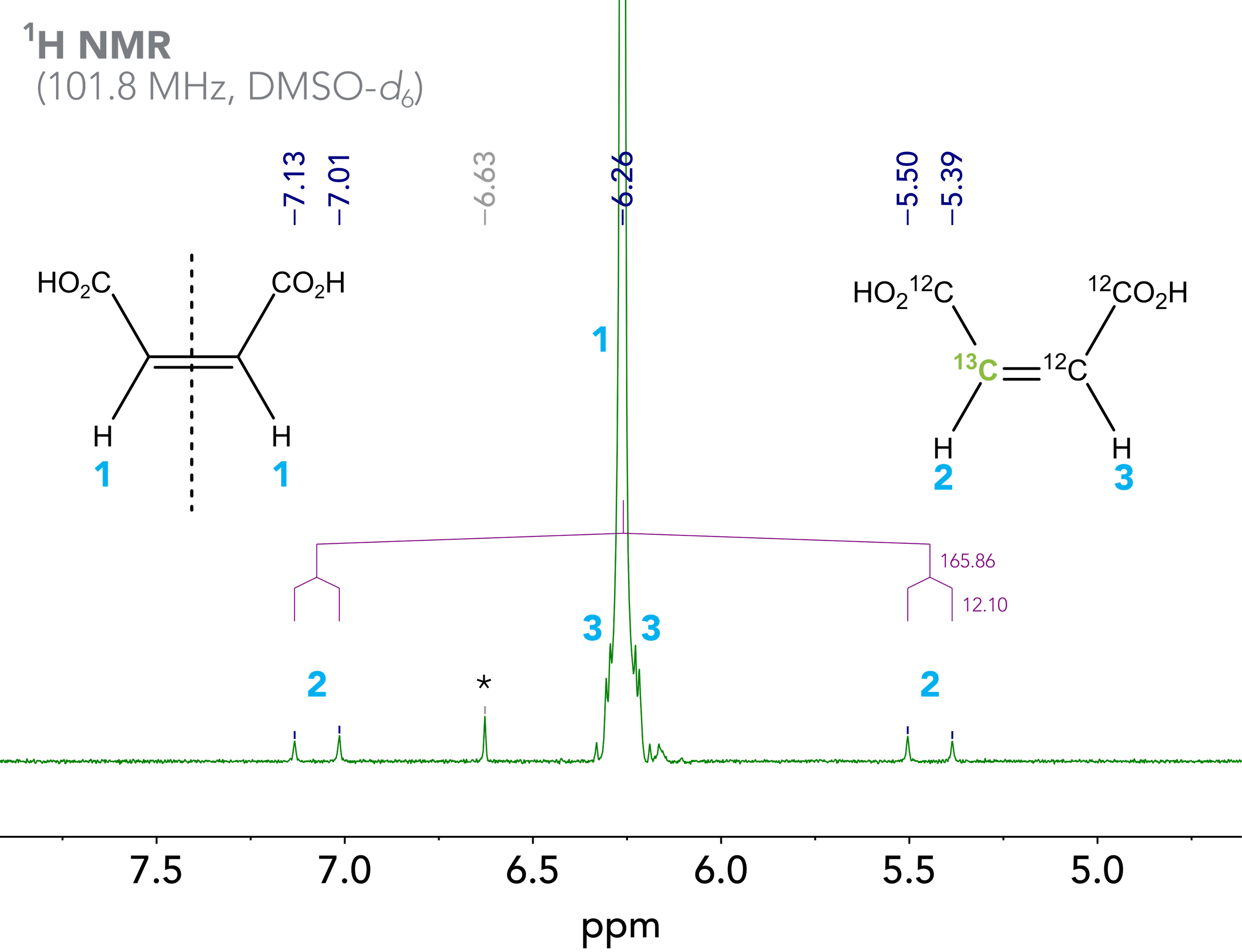
Figure 3. Zoomed view 100 MHz 1H NMR spectrum of maleic acid in DMSO-d6. The trans-isomer, fumaric acid, was observed as an impurity and indicated with an asterisk(*).
However, in the case of maleic acid, the satellite signals are not observed as a doublet (one “copy” of the main signal to its left and right side) as one would expect, but as doublet of doublets 2 with an additional coupling of 12.1 Hz. This extra coupling results from the fact that the 13C isotope of maleic acid (Figure 3, right) is not symmetric anymore like its 12C analogue, and the hydrogen atoms 2 and 3 are not chemically equivalent – so these protons couple with each other. The value of this J coupling agrees well with the typical range of 6-14 Hz for such vicinal cis couplings.1 This explains why the signal of the olefinic proton 2 appears as a doublet of doublets δ (101.8 MHz, DMSO-d6) = 6.29 (13CH, dd, 1JCH = 165.9 Hz, 3JHH = 12.1 Hz) ppm.
For the signal assigned to proton 3 we find even more peaks. We won’t go into details about those in this blog post to keep things relatively easy, but I think this shows that the NMR spectrum of maleic acid is a great example of how NMR spectroscopy is such an information rich analytical technique if you want it to be. The overview spectrum is as easy as a simple singlet, but if you look really closely, there are many more signals, and everyone tells their little story. Theoretical research groups, like the Physical Organic Chemistry Lab around Professor Tormena and Professor Rittner,2 help in rationalizing coupling constants by theoretical calculations, which proves that such signals are not simply some noise or artifact signals but do actually agree with NMR theory.
If you happen to work with maleic acid as an internal calibrant or at all, we hope this blog post helps you in finding an answer to the question why the carbon satellites for this compound look different from what one is used to. If you have any questions on benchtop NMR or interesting signals, please reach out to us, and we will be more than happy to help.
References
[1] LibreText CHEMISTRY https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_ Modules_(Organic_Chemistry)/Alkenes/Properties_of_Alkenes/Nuclear_Magnetic_Resonance_(NMR)_of_Alkenes (accessed 2023-09-22).
[2] Website of the Physical Organic Chemistry Lab at UNICAMP, Brazil https://pocl.iqm.unicamp.br/index.html (accessed 2023-09-15).